ELTE Eötvös Loránd University
1117 Budapest Pázmány Péter sétány 1/A, Hungary
room 637 extension 1108
room 638 extension 1109
room 643 extension 1909
e-mail: turanyi@chem.elte.hu

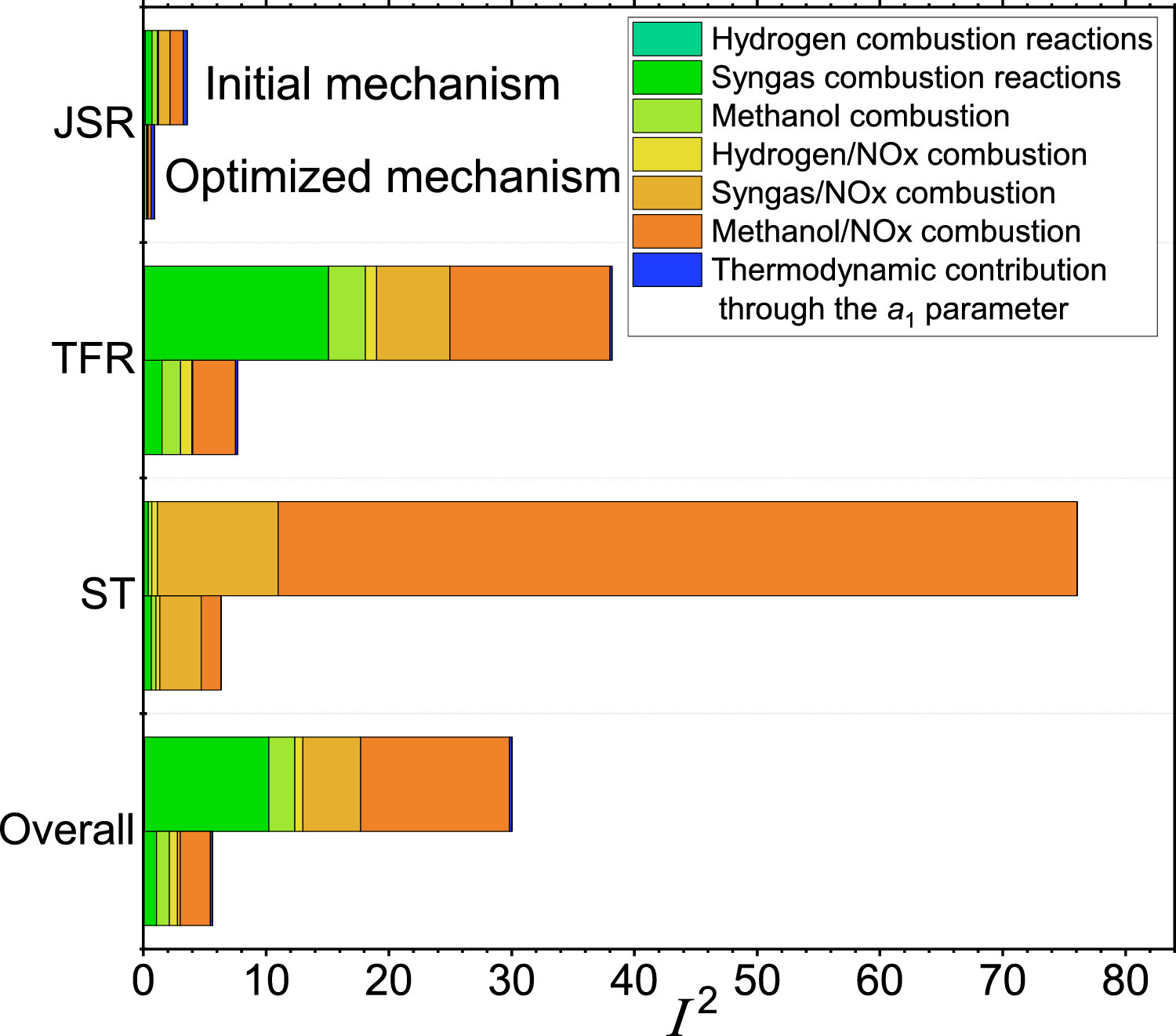
Uncertainty quantification of a newly optimized methanol/NOx combustion mechanism
Márton Kovács, Máté Papp, István Gy. Zsély, Tibor Nagy,
Tamás Turányi
Optima++ is a general framework for manipulating experimental data related to combustion chemistry, carrying out simulations of such experiments, performing model optimization and analysis, and providing auxiliary features for the above tasks. Optima++ is able to handle simulation codes Cantera, FlameMaster, OpenSMOKE++ and ZeroRK. Also, Chemkin Pro is coming soon.
An interactive web site, where the users may find Arrhenius parameters of gas phase elementary reactions determined in direct measurements, theoretical calculations or have been used in modelling studies. The users may recalculate the uncertainty limits of the rate coefficients. The editors have the right to upload data sheets for new reactions and to add, delete or modify existing data sheets. The editor status may be granted to any registered user upon request to the administrator.
Visit k-evaluation web page
Reaction fluxes of a combustion simulation can be visualized in the forms of still pictures and videos.
We maintain a collection of a series of Chemkin-format reaction mechanisms for the combustion of the following fuels:
hydrogen, syngas, methanol, ethanol, methane, butanol, fuels+NOx.




